Researchers at MIT and China’s Tsinghua University have created a “yolk and shell” battery which they believe could be charged from zero to full capacity in only six minutes.
The ‘egg’ batteries are made by creating an electrode consisting of nanoparticles with a titanium dioxide ‘shell’ and an aluminum ‘yolk’ for the anode. The electrodes in existing lithium batteries expand and shrink during each charge, which causes degradation over time. The aluminum filling of the newly designed nanoparticles would allow for increased growth which in turn causes less damage to their shells.
Not only are they more durable, but the new batteries are touted as being able to hold three times the capacity of current lithium-ion batteries found in mobile devices, meaning smartphones, laptops and tablets could go several days without requiring a recharge. Moreover, it’s claimed the lack of severe expand-contract performance degradation will allow the batteries to achieve a full charge within six minutes.
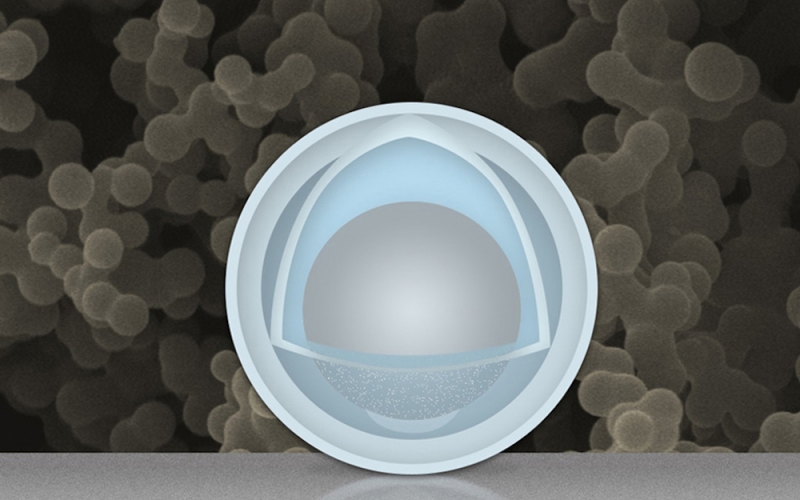
Presently, the batteries are still in the experimental lab stage, but researchers working on the project believe the simple method of construction and relatively low-cost of the materials could result in the invention’s mass commercial production.
“These yolk-shell particles show very impressive performance in lab-scale testing,”said David Lou, an associate professor of chemical and biomolecular engineering at Nanyang Technological University in Singapore,“to me, the most attractive point of this work is that the process appears simple and scalable.”
MIT professor Ju Li said the method behind the construction of the egg and yolk was a “chance discovery”, but one that could eventually see the technology become a rival to the current lithium-ion batteries used today.
https://www.techspot.com/news/61745-nanoparticle-yolk-shell-battery-could-charge-mobile-devices.html