Why it matters: Using silicon as the anode in lithium-ion batteries is something of a holy grail for researchers in the field. The energy storage capacity of silicon is 10 times greater than current graphite anodes, which can translate to double the capacity for the entire battery. The problem is that silicon is unstable, and so far, scientists have not come up with a way to produce pure silicon anodes.
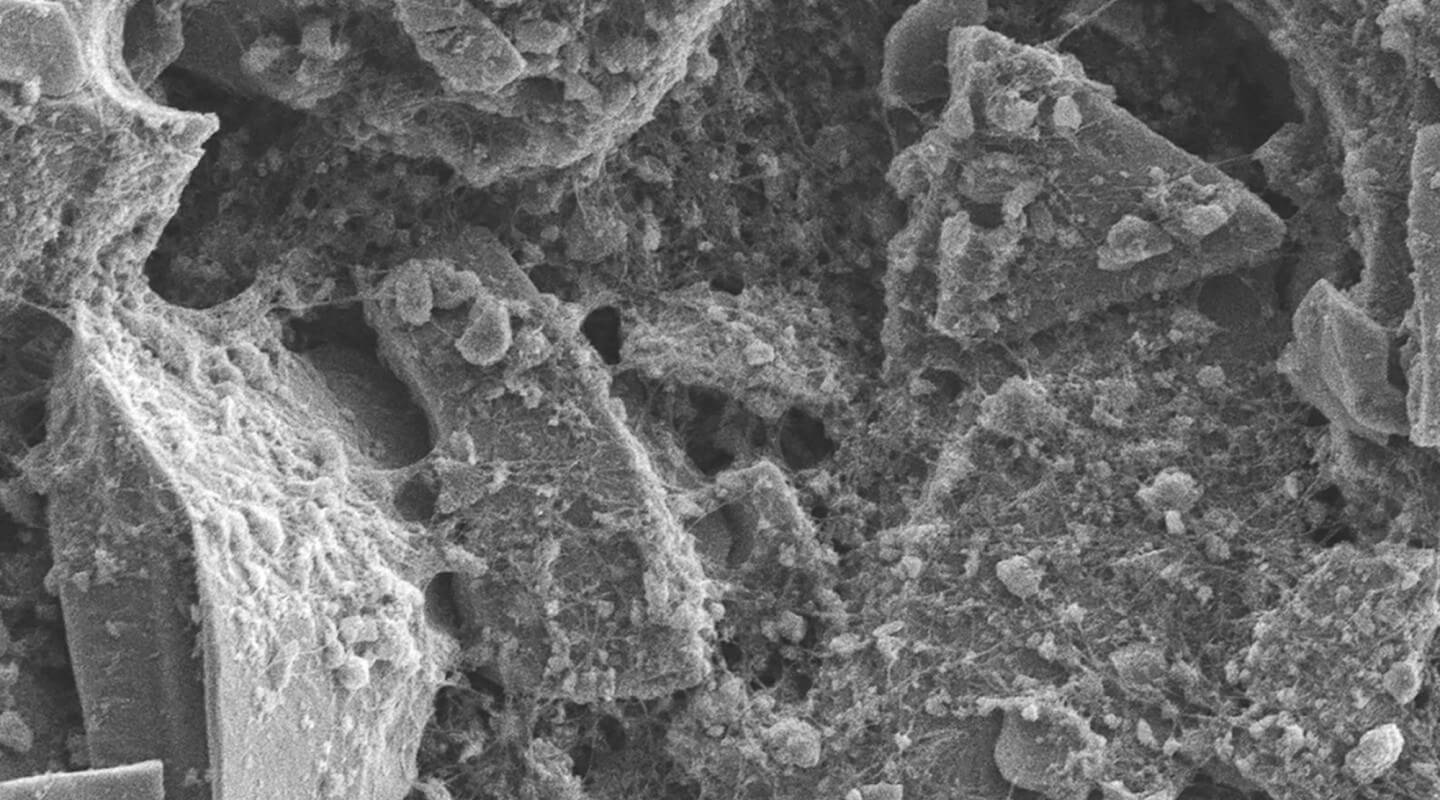
To help mitigate the reducing effects on silicon anodes, researchers at the University of Eastern Finland (UEF) have created a hybrid material consisting of mesoporous silicon (PSi) and carbon nanotubes (CNTs).
"The progress of the LIB (lithium-ion battery) research is very exciting, and we want to contribute to the field with our know-how related to mesoporous structures of silicon," said UEF Professor Vesa-Pekka Letho.
The PSi microparticles are made from the ash of barley husks using a process that extracts amorphous porous silicon from phytoliths, which are abundant in the ash. Using a process called "chemical conjugation," the team combines PSi and CNTs with the proper polarity so that the material does not hinder the defusion of lithium ions into the silicon. The scientists found by using the right conjugation method, both conductivity and durability of the anode was improved.
The team is continuing work aiming to develop a pure silicon anode with a solid electrolyte. It hopes to mitigate some of the inherent safety issues of lithium-ion batteries and unstable solid electrolyte interfaces.
"Hopefully, the EU will invest more in the basic research of batteries to pave the wave for high-performance batteries and to support the competitiveness of Europe in this field," said Letho. "The Battery 2030+ roadmap would be essential in supporting this progress."
If you're interested in the technical details, the team has published two papers on its process. "Conjugation with carbon nanotubes improves the performance of mesoporous silicon as Li-ion battery anode" was published in Scientific Reports back in March. "Cascading use of barley husk ash to produce silicon for composite anodes of Li-ion batteries" can be found in the April issue of Materials, Chemistry, and Physics.
Image credit: University of Eastern Finland