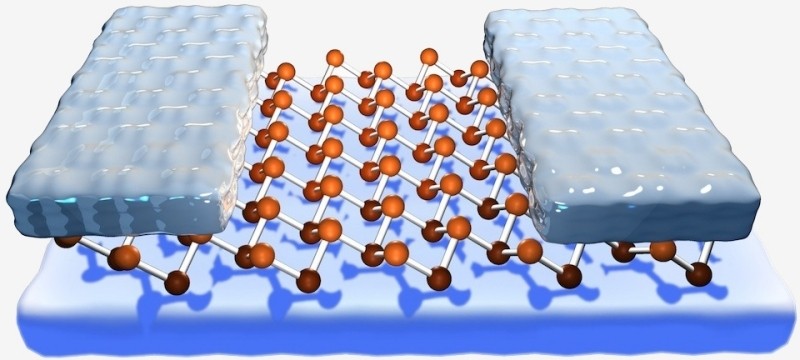
Graphene has been heralded as the successor to the modern-day silicon that powers our electronics but don’t count the venerable material out just yet. Researchers at the University of Texas at Austin’s Cockrell School of Engineering have managed to create a transistor using silicene, the silicon version of graphene that’s just one atom thick.
Silicene is a volatile material that disintegrates when it reacts with oxygen in the air. As such, the research team built a sheet of it on a thin silver surface. It was then capped with aluminum oxide and by gently scraping away some of the aluminum, they were able to create source and drain contacts resulting in a field effect transistor.
Hopes were high as electrons flowed through the silicene but the end results were a bit disappointing. Scientists and researchers had predicted that electrons would flow through the material much like they do with graphene but that simply wasn’t the case.
Instead, they observed electron mobility that was about 10 times less than expected. It’s unclear just yet if this is directly related to the silicene itself or perhaps a fault with their production technique.
There’s still plenty of research that needs to take place before determining if silicene could overthrown graphene. Even still, it’s a pretty significant milestone. Assistant professor Deji Akinwande said the major breakthrough here is the efficient low-temperature manufacturing and fabrication of silicene devices for the first time.
https://www.techspot.com/news/59659-researchers-build-one-atom-thick-silicene-transistor.html