Researchers at Yonsei University in Seoul, South Korea, have developed a new method of treating graphene that could lead to smaller and more efficient batteries.
Graphene is the most conductive material known to man but its thin and flat nature limits its surface area. That’s a problem when it comes to battery power as you want as much surface area as possible. The solution is to somehow create more surface area which is exactly what the South Korean researchers have done.
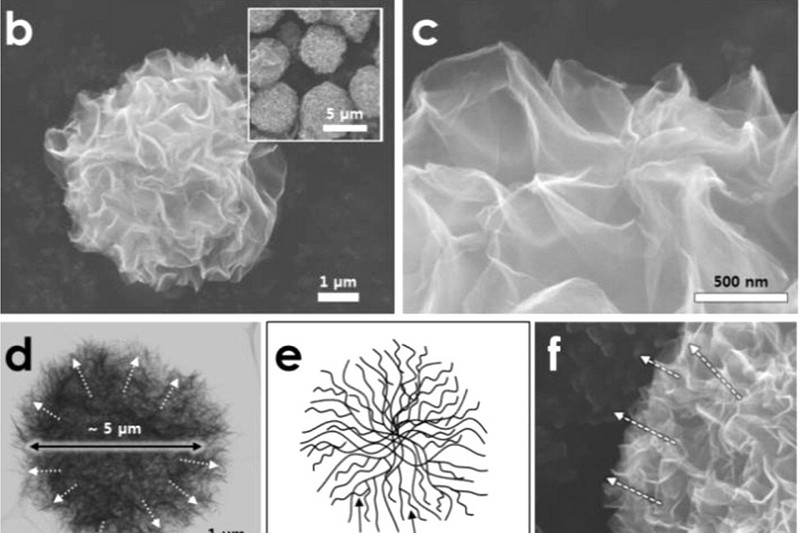
By using an ultrasonic nozzle, the researchers were able to create microdroplets of graphene oxide. These microdroplets were then dropped into a piping hot (160 C) mixture of organic solvent and reducing agent, much like one would dip chicken into hot oil to create deep-fried chicken.
This process extracts the graphene out from the center of the microdroplets to create three-dimensional graphene pom-poms with a wealth of surface area that’s ripe for battery applications. Early testing shows promise as the graphene pom-poms performed better as electrodes compared to regular sheets of graphene.
It’s worth pointing out that this isn’t the first time someone has come up with a method to create 3D graphene. What’s noteworthy about this technique, however, is the fact that it’s much easier to replicate on a large scale meaning mass production for use in consumer goods would be much more feasible.
While it’ll still be a while before graphene is powering our gadgets and electric cars, this breakthrough certainly puts us one step closer to that reality.
https://www.techspot.com/news/59448-deep-fried-graphene-could-key-long-lasting-batteries.html