In a nutshell: Researchers from the Korea Advanced Institute of Science and Technology (KAIST) have discovered a promising battery breakthrough that could revolutionize portable power. Professor Jeung Ku Kang and his team's work involves sodium-ion batteries, which are similar in design to traditional lithium-ion batteries and can be manufactured using the same sort of industrial processes. In these batteries, sodium ions replace lithium ions in the cathode, while the lithium salts in the electrolyte (the liquid that helps ferry charge between the battery electrodes) are traded out for sodium salts.

Sodium-ion batteries aren't new, but they've only started to gain traction in recent years. Compared to their lithium counterparts, the materials used in sodium batteries are far more abundant (up to 1,000 more plentiful) and affordable. They are also much safer than lithium-ion batteries, and can be discharged to 0V – eliminating the possibility of thermal runaway due to a short circuit.
Long charge times and less than desirable storage capacity, however, have kept sodium-ion batteries on the sidelines, but that could all change soon.
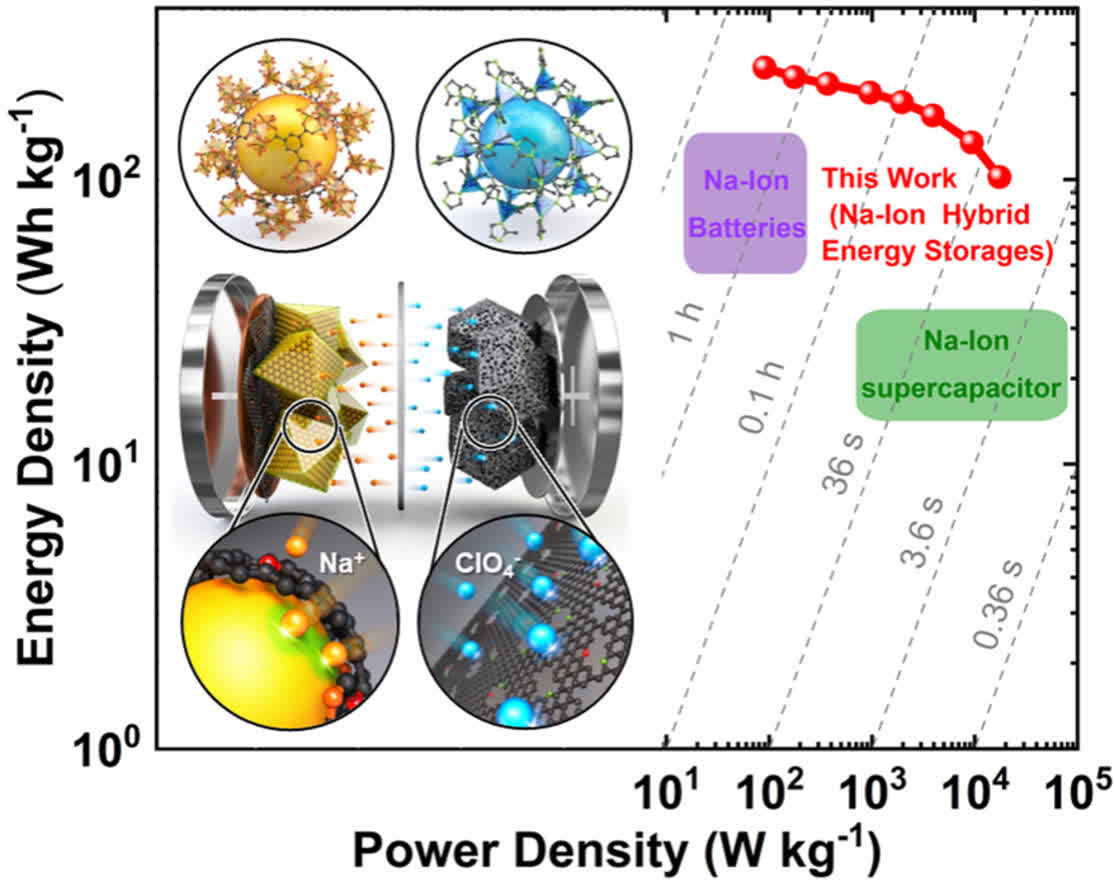
The KAIST team replaced common battery cathode materials with those used by supercapacitors, resulting in a high-energy, high-power hybrid sodium battery that can also be charged rapidly. Tweaks to the anode were made to improve capacity, and a method to synthesize an optimized electrode material was also utilized.
According to Kang, their solution has an energy density that exceeds commercially available lithium-ion batteries along with the output density characteristics of a capacitor. As a next-gen storage device, recharging will be possible in seconds to minutes, which would make it ideal for use in all sorts of electronic gadgets. It could also be a game-changer for automakers building EVs, allowing them to cut costs while delivering vehicles that could fully recharge in just a couple of minutes.
With any luck, the tech will eventually make its way from the lab to the real world.
The team's research has been published in the journal Science Direct.
Image credit: Mike Bird