Forward-looking: Researchers from RMIT University in Australia have developed a new type of battery that replaces hazardous electrolytic fluids with water. Further research and development is needed, but the potential for safer alternatives to lithium-ion batteries and greener alternatives to lead-acid batteries now exists.
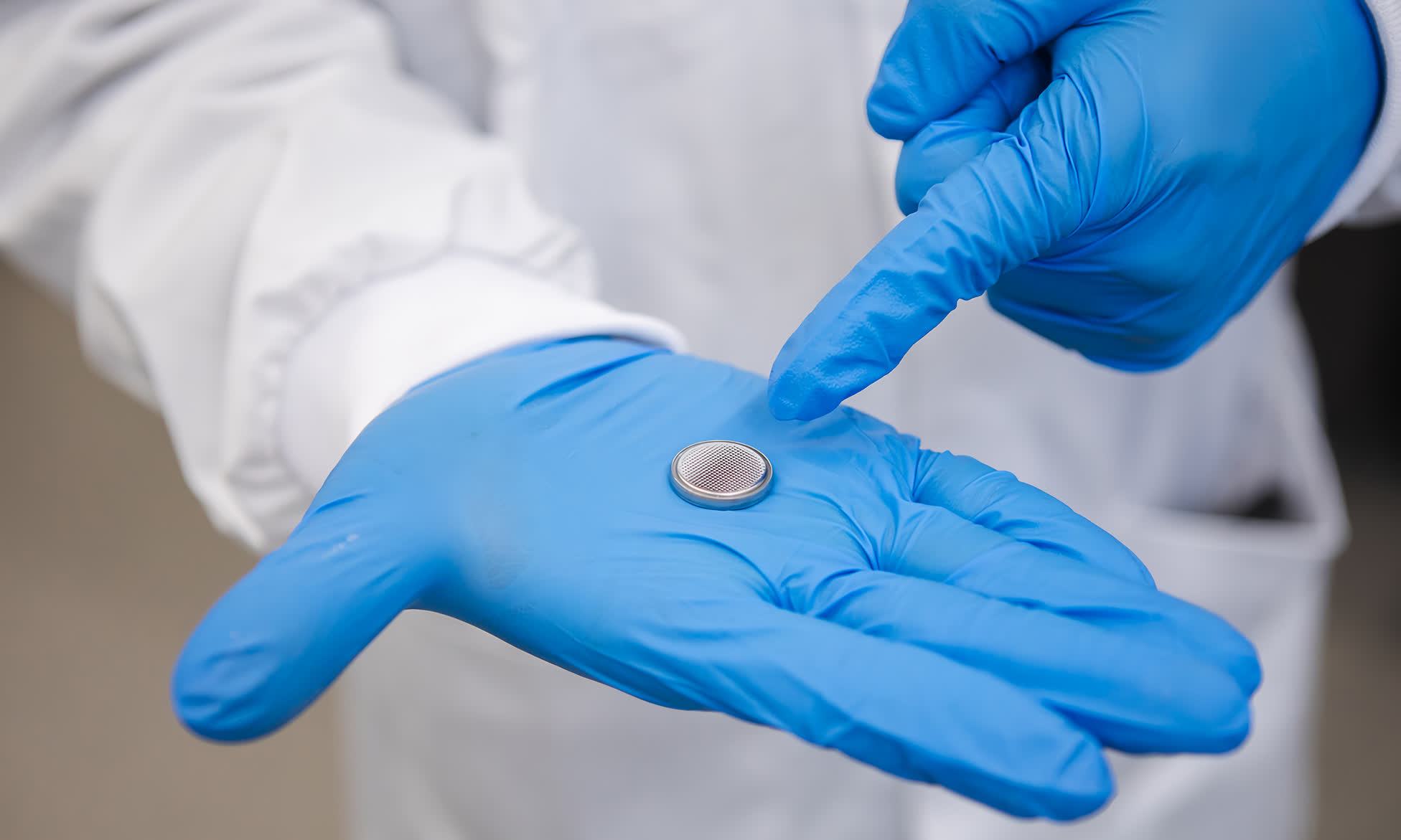
The team's liquid battery uses standard water with a few added salts in place of traditional electrolytic fluid like sulfuric acid or lithium salt to enable the flow of current between the positive and negative ends of the battery. It also uses bismuth metal as a coating for the zinc anode, which acts as a protective later to stop dangerous dendrites from forming, and magnesium for improved energy density.
In early testing, the water battery was able to retain 85 percent of its capacity after 500 charge cycles. Prototypes developed thus far include coin-sized batteries and cylindrical versions resembling traditional AA and AAA batteries. A design was also tested that connected to a solar panel and a 45-watt solar light that was able to keep it illuminated for 12 hours following a full day's charge.
Project lead Tianyi Ma, a chemical scientist at RMIT University, said the simplicity of their manufacturing process will make mass production that much easier. Their batteries will also be able to be safely disassembled for reuse or recycling, addressing end-of-life disposal challenges that plague current energy storage technology.
As seems to be the case with virtually every lab-based battery advancement that comes down the pipe, this one is far from being ready for prime time. The researchers are actively working to improve the energy density of their battery design by creating new nano materials to use as the electrode, in an effort to make them more comparable to lithium-ion batteries commonly found in consumer electronics.
Ma believes the water battery has the potential to replace lead-acid batteries within one to three years, and lithium-ion batteries within five to 10 years.
The team's study has been published in the science journal Advanced Materials.
https://www.techspot.com/news/102176-eco-friendly-water-batteries-cheaper-safer-than-lithium.html
