A hot potato: Despite Covid-19 cases continuing to rise, companies are taking different approaches when it comes to employees returning to the office. Some have already called staff back, while others are sticking with a remote work policy until at least next year. In Netflix’s case, CEO Reed Hastings says his workers won’t be returning until the “majority” are vaccinated.

In an interview with the Wall Street Journal, Hastings let it be known that he’s not a fan of working from home, believing that a lack of in-person interaction negatively impacts business. He was also asked whether companies will shift more towards working from home once the Covid-19 pandemic is over.
“If I had to guess, the five-day workweek will become four days in the office while one day is virtual from home. I’d bet that’s where a lot of companies end up,” said Hastings.
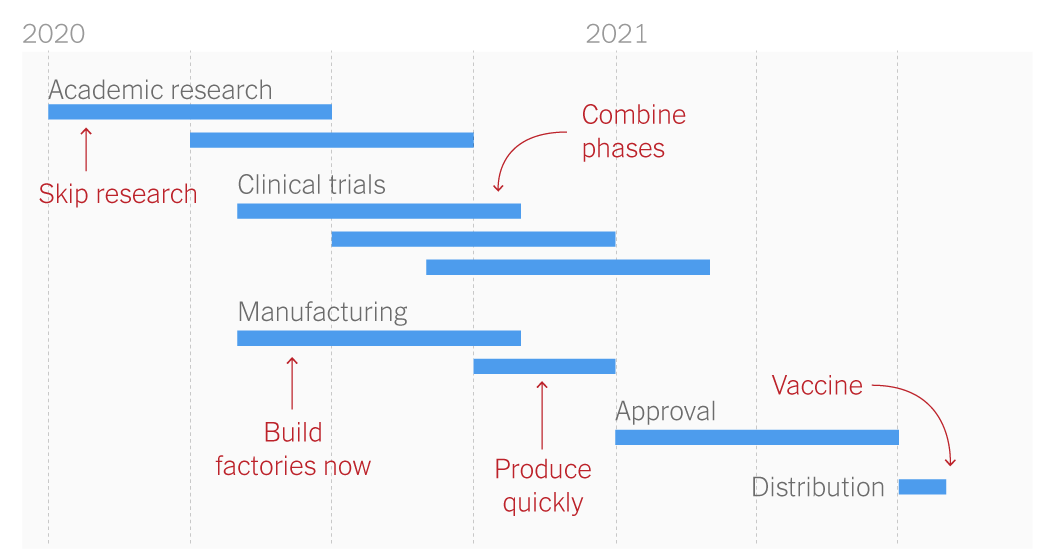
Vaccine development timeline, courtesy of The New York Times
The CEO was asked when his own workforce will return to the office. Interestingly, his plan is to wait until after a vaccine has arrived. “It’s probably six months after a vaccine. Once we can get a majority of people vaccinated, then it’s probably back in the office.”
Most scientists put the arrival of a Covid-19 vaccine sometime next year, though the bigger problem might be convincing people to take it; many fear a rushed development and testing time means the vaccine could be unsafe. Hastings said he wants “a majority” of people vaccinated before an office return, so it’s unlikely to be mandatory.
Most big tech firms have set 2021 as the date for worker returns. Microsoft isn’t opening its physical offices until next year, while Google said in July that employees could work from home for an additional 12 months. Amazon has also extended its remote working policy until January 8, 2021. Apple, however, began the first phase of reintroducing staff to its Silicon Valley HQ on June 15.
Image credit: JHVEPhoto
https://www.techspot.com/news/86667-netflix-boss-staff-can-return-office-when-majority.html